To follow on from last week’s post on grid computing in our series on the technological applications of astronomy to society we have an article on spectroscopy and the discovery of a new element in the cosmos – helium – by Armagh Observatory and Planetarium’s Michael Burton. He is our Director and before coming to Armagh was the Director of Teaching at the School of Physics in the University of New South Wales in Australia. He is also a member of the International Astronomical Union and President of the IAU’s Division B (“Facilities, Technology, Data Science”).
An entirely new element was discovered by astronomers in the 19th century when applying the then nascent diagnostic tool of spectroscopy to the exploration of the cosmos. Helium, unknown on the Earth at that time, was discovered in a spectrum taken of the light from the Sun.
The two most abundant elements in the Universe are the two lightest elements, hydrogen and helium. They make up the vast majority of the mass of the observable universe, dwarfing the contribution from all heavier elements combined by a wide margin. Helium is the second lightest, as well as the second most abundant element. It makes up nearly a quarter of the mass contained in the stars. Stars are indeed helium factories. They spend most of their lives creating helium from the hydrogen in their cores, through the process of nuclear fusion. Yet Helium was unknown as an element until the middle of the 19th century. It was only discovered by chance through the application of the new tool of spectroscopy to observations of the light from the Sun, enabling the splitting up of the light into its component colours.
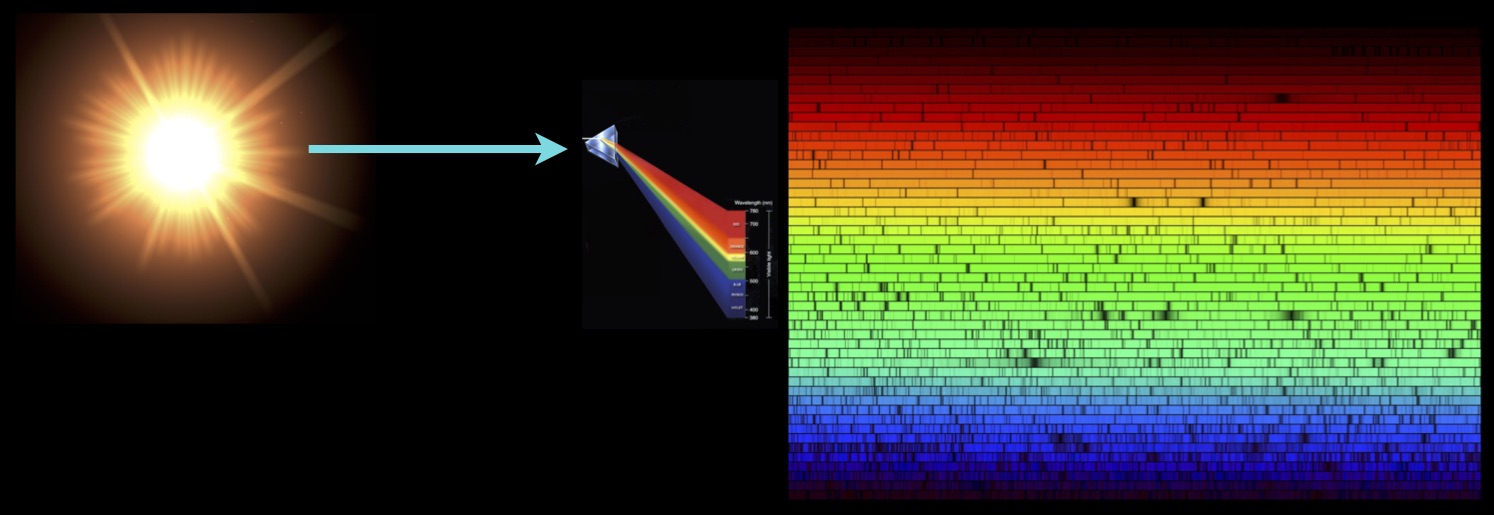
In 1868 astronomer Jules Janssen observed a solar eclipse in India through a prism. He noticed a bright yellow spectral line emanating from solar prominences – giant storms erupting from the Sun. The line was unidentified. At first it was thought to come from the chemical element sodium since it was close by to two other bright lines arising from this element. The two lines of sodium were known as the D1 and D2 Fraunhofer lines, named after their discoverer. The unknown line was identified later that year by astronomer Norman Lockyer in a solar spectrum and he named it the D3 Fraunhofer line. He concluded it was caused by a new, unknown element. Lockyer named it Helios, after the Greek word for the Sun. Helium was then unknown, and in fact was not discovered on the Earth for another 27 years, until 1895.
Several fascinating properties of helium have been discovered in the ensuing years. The alpha particle, produced through radioactive decay, was found to be a helium nucleus. It is the most basic product of nuclear fusion in stars, as protons are turned into helium nuclei. Helium, when cooled to near absolute zero, is a liquid and has no viscosity, a phenomenon we call superfluidity.

Helium is now used in a wide range of important industrial applications. The most well-known is flight, where helium gas (being less dense than air) naturally provides buoyancy for airships and balloons. Owing to its low boiling point helium is used extensively in cryogenic applications. Liquid helium acts as a coolant for superconducting “magnetic resonance imaging” (MRI) scanners used for medical diagnosis.



0 Comments